There are in all six pharma companies, showing a ray of hope towards vaccine development for covid-19. Apart from three Chinese clinical trials, these are the other three pharma companies to enter phase 3 of clinical trial .
About the Oxford COVID-19 vaccine:
The Oxford team had already used ChAdOx1 vaccine technology to produce candidate vaccines against a number of pathogens including flu, Zika and Middle East Respiratory Syndrome (MERS), another coronavirus. The ChAdOx1 vaccine is a chimpanzee adenovirus vaccine vector. This is a harmless, weakened adenovirus that usually causes the common cold in chimpanzees. ChAdOx1 was chosen as the most suitable vaccine technology for a SARS-CoV-2 vaccine as it has been shown to generate a strong immune response from one dose in other vaccines.
It has been genetically changed so that it is impossible for it to grow in humans. This also makes it safer to give to children, the elderly and anyone with a pre-existing condition such as diabetes. Chimpanzee adenoviral vectors are a very well-studied vaccine type, having been used safely in thousands of subjects.
Coronaviruses have club-shaped spikes on their outer coats, which form a corona – Latin for crown – on the virus surface. Immune responses from other coronavirus studies suggest that these spikes are a good target for a vaccine.About Pfizer and BioNTech mRNA Vaccine:
After extensive review of preclinical and clinical data from Phase 1/2 clinical trials, and in consultation with the U.S. Food and Drug Administration’s Center for Biologics Evaluation and Research (CBER) and other global regulators, Pfizer and BioNTech have chosen to advance their BNT162b2 vaccine candidate into the Phase 2/3 study, at a 30 µg dose level in a 2 dose regimen. BNT162b2, which recently received U.S. Food and Drug Administration (FDA) Fast Track designation, encodes an optimized SARS-CoV-2 full length spike glycoprotein (S), which is the target of virus neutralizing antibodies.
In the preclinical studies, BNT162b1 and BNT162b2 candidates induced favorable viral antigen specific CD4+ and CD8+T cell responses, high levels of neutralizing antibody in various animal species, and beneficial protective effects in a primate SARS-CoV-2 challenge model.
BNT162b2 vaccinated human participants displayed a favorable breadth of epitopes recognized in T cell responses specific to the SARS-CoV-2 antigen, as compared to the BNT162b1 candidate. BNT162b2 demonstrated concurrent induction of high magnitude CD4+ and CD8+ T cell responses. BNT162b2 elicited T cell responses against the receptor binding domain (RBD) and against the remainder of the spike glycoprotein that is not contained in the BNT162b1 vaccine candidate. The companies believe that immune recognition of more spike T cell epitopes may have the potential to generate more consistent responses across diverse populations and in older adults.About Moderna mRNA-1273 vaccine:
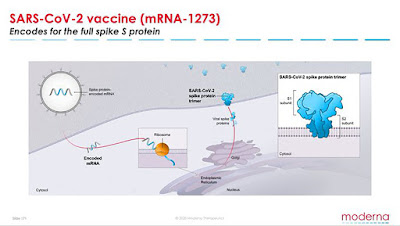
mRNA-1273 is an mRNA vaccine against COVID-19 encoding for a prefusion stabilized form of the Spike (S) protein, which was co-developed by Moderna and investigators from NIAID’s Vaccine Research Center. The first clinical batch, which was funded by the Coalition for Epidemic Preparedness Innovations, was completed on February 7, 2020 and underwent analytical testing; it was shipped to NIH on February 24, 42 days from sequence selection.
The first participant in the NIAID-led Phase 1 study of mRNA-1273 was dosed on March 16, 63 days from sequence selection to Phase 1 study dosing. On May 12, the FDA granted mRNA-1273 Fast Track designation. The potential advantages of an mRNA approach to prophylactic vaccines include the ability to combine multiple mRNAs into a single vaccine, rapid discovery to respond to emerging pandemic threats and manufacturing agility derived from the platform nature of mRNA vaccine design and production.



No comments:
Post a Comment